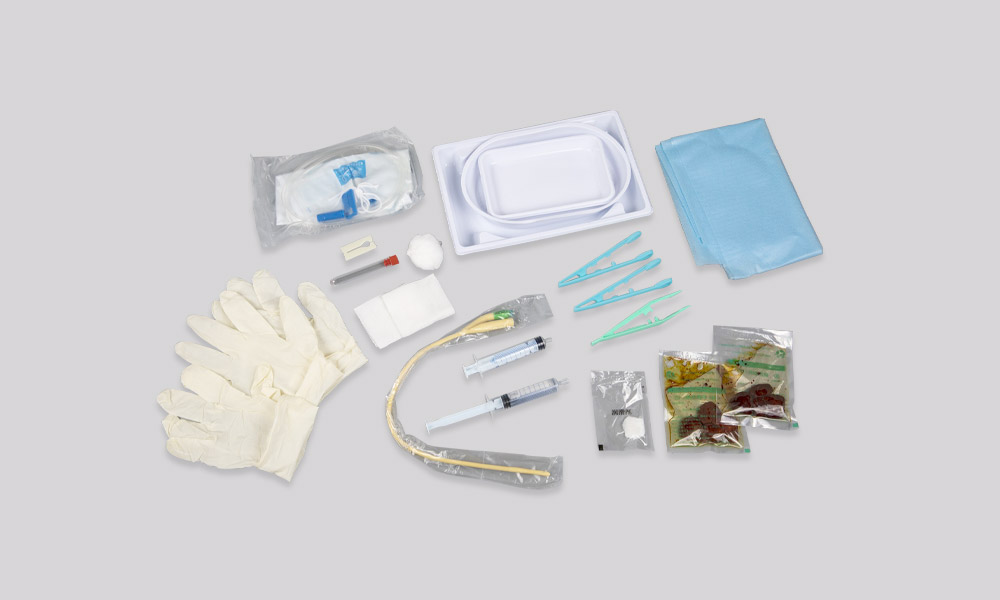
[Product Name] Disposable surgical dressing kits
[Registration Certificate Number] Zhejiang Machinery Registration Approval 20142140008
[Registration Certificate Validity Period] September 17, 2024 to September 16, 2029
[Performance Structure] This product has been sterilized with ethylene oxide and is a sterile product; When the product leaves the factory, the residual amount of ethylene oxide should not exceed 10mg/kg; Surgical drapes, surgical drapes (small), sterile protective covers, surgical gowns, patient clothing, patient pants, clean bags, treatment drapes, gauze blocks, specimen bags, medical surgical masks, surgical caps, foot covers, surgical sutures, medical suture needles, sterilized rubber surgical gloves or rubber examination gloves, lumbar discs, tweezers, measuring cups, plastic basins, cotton balls, iodine swabs, cotton swabs, water dispensers, abdominal bands, middle sheets, U-shaped sheets, cotton pads, gauze bandages, alcohol cotton balls, medical tapes, sterile surgical films, elastic bandages, sterile plastic handle surgical knives, hemostatic bands, disinfection brushes, medical forceps, small towels, sterile surgical blades, fluid bags, scissors, circumference sheets, Composed of sleeve covers, leg covers, inner wrapping fabric, and outer wrapping fabric. The contents can be determined according to the requirements of the user contract, provided that the intended use of the product is not changed and the contents do not exceed the specified range. The specification tolerance is ± 8%.
[Scope of Application] Used as a supporting tool during surgeries in medical institutions.
[Usage period] Under the condition of complying with storage and transportation rules, the validity period is two years from the date of sterilization.



 English
English 简体中文
简体中文